New Pneumococcal Polysaccharide Conjugate Vaccine Synflorix Added To The National Immunisation Schedule. All comparisons led to the same conclusion that the threshold defined as. Pneumococcal polysaccharide conjugate vaccine (adsorbed).
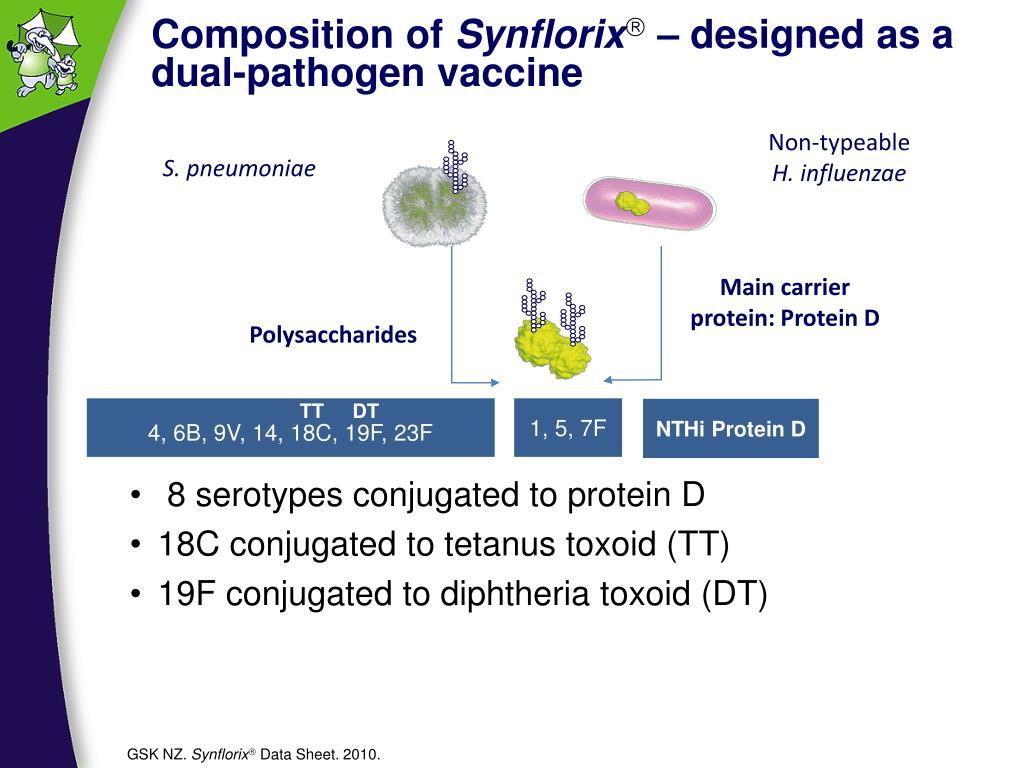
Conjugate vaccines and polysaccharide vaccines.
Pneumococcal polysaccharide vaccine children in this age group do not develop an effective immune response to the capsular types contained in this polysaccharide vaccine. Pneumococcal polysaccharide vaccine vaccine description target disease streptococcus pneumoniae type conjugate vaccine clinical schedule of vaccination. Antigen literature review for the new zealand national immunisation schedule, 2016: Infants concomitantly received the routine epi vaccinations according to the schedule in the gambia (appendix p 5).
Post a Comment for "New Pneumococcal Polysaccharide Conjugate Vaccine Synflorix Added To The National Immunisation Schedule"